The C-cl Bond Dissociation Energy in Cf3cl
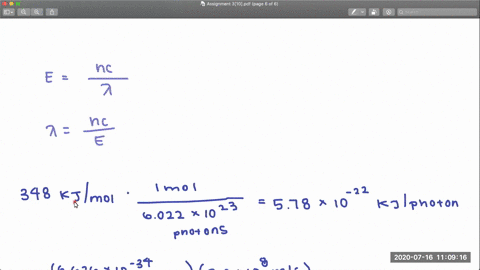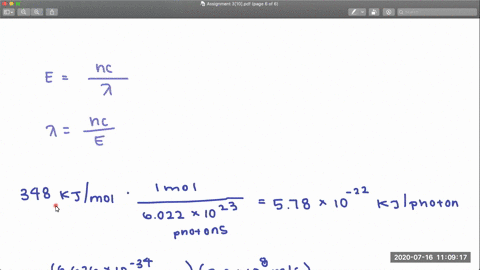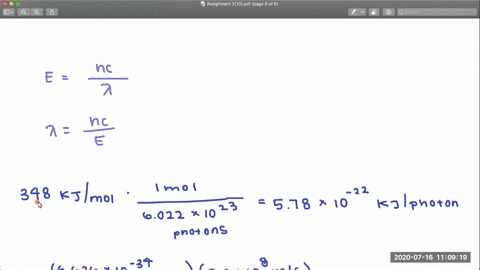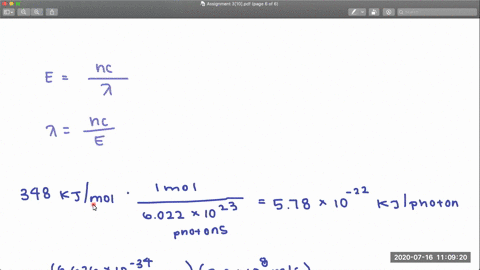
The dissociation energy of the C-Cl bond in CF3Cl is 339 kJmol. The effects of the Cl equatorial belt present in perhalogenated molecules such as CF3Cl have been hitherto overlooked in describing the origin of noncovalent interactions.

Calculate C Cl Bond Enthalpy From Following Reaction Ch3cl G Cl2 G Ch2cl2 G Hcl G Dh O 104kj If C H Cl
Part A What is the range of wavelengths of photons that can cause CCl bond rupture in one molecule but not in the other.
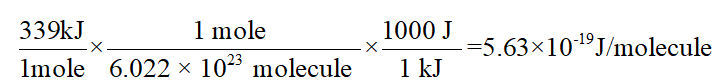
. The coincident time-of-flight mass spectra and three-dimensional time-sliced images of the CF 2 Cl fragment were recorded at a few specific photon energies. 353 nm 248 nmBThe ionization energy of O2 is 1205 kJmolO2 hv -----. Vertical excitation energies DeltaEvertical dissociation energies DeltaEdiss dissociation enthalpies DeltaHdiss and the oscillator strength f have also been computed.
O2 e-The maximum wavelength of light Question. We herein report a dataset of 28 homolytic CCl bond dissociation energies BDEs to be known as the CCl28 dataset obtained using the benchmark-quality W1w thermochemical protocol. CCl for CCl bond cleavage on the Pd111 surface.
353 nm 248 nm. Department of Energys Office of Scientific and Technical Information Ab Initio Calculations and Three Different Applications of Unimolecular Rate Theory for the Dissociations of CCl4 CFCl3 CF2Cl2 and CF3Cl Journal Article OSTIGOV. With increase in p character in an orbital bond length will increase while with increase in s character in an orbital bond length will decrease.
What is the range of wavelengths of photons that can cause CCl bond. In CF3Cl the C-Cl bond-dissociation energy is 339 kJ mol. Vertical ionization energies of the CF 3Cl molecule were derived tobe1246 and1310eVrespectivelyandtheverticalionization energies of the A2A 1B 2A 2C 2E and D2E states were 150 155 165 and 174 eV respectively81023 Once the internal energy exceeds the dissociation limits the CCl and CF bond cleavages of CF 3Cl may.
This set contains chlorinated organic molecules that consist of either sp3 - sp2 - or sp -hybridized CCl bonds. Basically I already answered this but I was a bit confused about the result cause I dont know if there is a bond that is negative cause am familiar with the energy given to dissociation of a bond or the formation of it but here its given the energy to the formation of chlorine and carbon I dont know if thats the same as the. Founded upon the knowledge that carbon-chlorine bond dissociation energies BDEs for awide range of a-chloro carbonyls fall within anarrow range eg74kcalmol1 for chloroacetic acid to 77 kcalmol1 for chloroacetamides7 In addition these CCl systems are expected to have bond polarization characteristics that are similar with respect to.
The rotational spectra of two isotopologues CF 335 ClCO and CF 337 ClCO of the CF 3 ClCO adduct have been investigated and analyzed using supersonic-jet Fourier transform microwave spectroscopy and found to have the features of a symmetric top. Lee and Sudarshan Guha. In CF 3 Cl the CCl bond-dissociation energy is 339 kJmol.
Bond energy is the energy required to break or the energy released upon making of a mole of that bond. Rotational centrifugal distortion and nuclear quadrupole 35 Cl and 37 Cl coupling constants have been. In CCl 4 the CCl bond-dissociation energy is 293 kJmol.
The CO bond dissociation energy in CO2 is 799 kJmol. What is the maximum wavelength of photons that can rupture this bond. The dissociative photoionization of CF 3 Cl was investigated using threshold photoelectron photoion coincidence TPEPICO imaging in the energy range of 12301850 eV.
Full geometry optimizations have been carried out for S0 as well as relaxed potential energy calculations for both states along the C-Cl bond distance. The CCl and CF bond dissociation energies in CF3Cl are 339 kJmol and 482 kJmol respectively. Dissociation of the CCl bond was observed by using X-ray photoe-mission spectroscopy XPS to monitor the presence of chlorine on the Pd111 surface after heating.
Besides its typical halogen donor behavior exhibiting a Cl σ-hole in forming ClB halogen bonds B is an electron-rich region CF3Cl reveals a new interaction site in its complex with CO2 when. The 1E des for each alkyl chloride was obtained from temperature programmed desorption TPD experiments. It more or less dictates how strong or weak the given bond is.
A comparison of bond dissociation energies of the type D CF 3 X from studies of equilibria with those obtained by. The maximum wavelength of light that has enough energy per photon to dissociate the. Answer 1 of 2.
PDF The dissociative photoionization of CF3Cl was investigated using threshold photoelectron photoion coincidence TPEPICO imaging in the energy. The reaction CH4g Cl2--- CH3Clg HCl g has H -25kcal and bond dissociation energy of C-Cl H-Cl C-H and Cl-Cl is given as 84 kcalmol 103. AThe C-Cl and C-F bond dissociation energies in CF3Cl are 339 kJmol and 482 kJmolrespectively.
What is the range of wavelengths of photons that can cause C-Cl bond rupture in one molecule but not in the other. In CCl4 the bond-dissociation energy is 293 kJ mol. The c-cl bond dissociation energy in cf3cl is 339 kjmol.
Generally ionic bonds are stronger than covalent bonds. Concise Inorganic Chemistry for JEE by JD. In CCl4 the CCl bond-dissociation energy is 293 kJmol.
For example d C C l in C H X 3 C l Å 178 Å d C C l in C F X 3 C l Å 175 Å. In CF3Cl the CCl bond-dissociation energy is 339 kJmol. The maximum wavelengths of electromagnetic radiation required to rupture these bonds are ________ and ________ respectively.
These enthalpy changes are combined with other data to give D CF 3 Cl 86108 D C 2 F 5 Cl 82717 and Δ Hf CF 3 Cl169410 all in kcal mole 1 at 298K. The species in this set have CCl BDEs at 298 K. Up to 256 cash back 28 Mar 2020.
These results are compared with other published values of D CF 3 Cl and Δ Hf CF 3 Cl.
Solved In Mathrm Cf 3 Mathrm Cl The Mathrm C Mathrm Cl Bond Dissociation Energy Is 339 Mathrm Kj Mathrm Mol In Mathrm Ccl 4 The Mathrm C Mathrm Cl Bond Dissociation Energy Is 293 Mathrm Kj Mathrm Mol What Is The Range
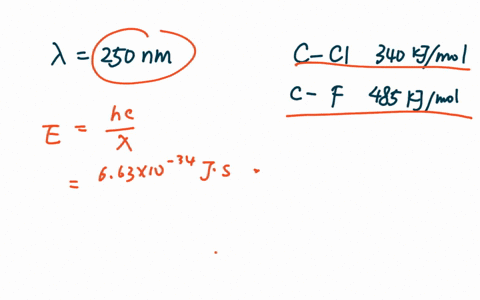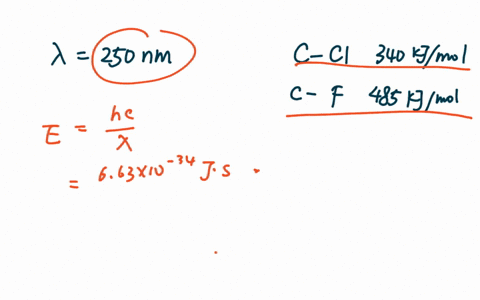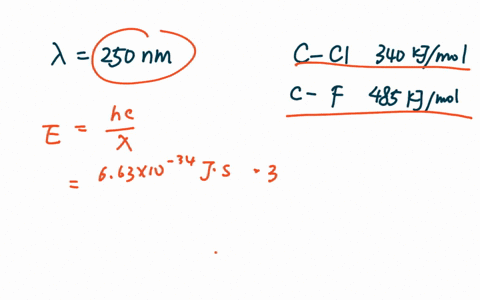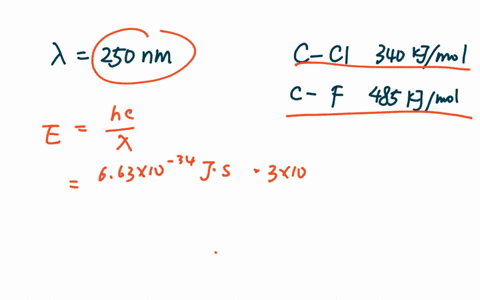
Solved In Mathrm Cf 3 Mathrm Cl The Mathrm C Mathrm Cl Bond Dissociation Energy Is 339 Mathrm Kj Mathrm Mol In Mathrm Ccl 4 The Mathrm C Mathrm Cl Bond Dissociation Energy Is 293 Mathrm Kj Mathrm Mol What Is The Range
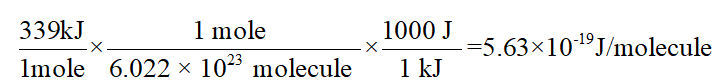
Answered In Cf3cl The C Cl Bond Di S Sociat Ion Bartleby

Solved In Cf3cl The C Ci Bond Dissociation Energy Is 339 Chegg Com
Comments
Post a Comment